Which of the Following Should Have the Highest Boiling Point
Hence we can say that more number of ions will increase the boiling point of solution. The Na2CO3 solution has a higher osmotic pressure and higher boiling point than the LiCl solution.

Which Of The Following Will Have The Highest Boiling Point At 1 Atm Pressure Youtube
Which of the following will have the highest boiling point.

. Which of the following aqueous solutions should have the highest boiling point. Because the boiling point of molecules of similar size depends on the differences in functional groups of the molecule. B As we know greater the value of vant Holf fctor higher will be the elevation in boiling point and hence higher will be the boiling point of solution.
Substance Molecular Mass amu Dipole Moment Propane CH3CH2CH3 44 01 Dimethylether CH3OCH3 46 13 Methylchloride CH3Cl 50 19 Acetaldehyde CH3CHO 44 27 Acetonitrile CH3CN 41 39. Which of the following should have the highest boiling point. Which of the following should have the highest boiling point.
10 M NA 2 SO 4 d. 10 M NaOH c. Na2SO4 have ions with charge -2.
CCl 4 CBr 4 C. Which of the following should have the highest boiling point. Which of the following should have the highest.
A H2O B H2S C HCl D CH4 E CH3C OH Acetaldehyde The post Which one of the following should have the highest boiling point. Solution for Which of the following should have the highest boiling point A CH3-CH2-F B CH3-CH2-CH3 C CH3-CH2-CH2-CH3 D CH3-CH2-O-H. A Cas b CH3OCH3 c BC13 d XeFsCI 6.
Ionic molecules have higher boiling point than covalent molecules. This is the best answer based on. So the answer is C.
This problem has been solved. Which of the following should have the highest boiling point. They all have the same boiling point.
Based on molecular mass and dipole moment of the five compounds in the table below which should have the highest boiling point. Which one of the following should have the lowest boiling pointPH3 H2S HCl SiH4 H2O. Should have the highest boiling point.
In comparison to a 001 M solution of glucose the depression in freezing point of a 001 M MgCl solution is _____. Substance Molecular Mass amu Dipole Moment D Propane CH3CH2CH3 44 01 Dimethylether CH3OCH3 46 13 Methylchloride CH3Cl 50 19 Acetaldehyde CH3CHO 44 27 Acetonitrile CH3CN 41 39 ACH3CN BCH3CH2CH3 CCH3OCH3 DCH3Cl ECH3CHO 1 2Of the following substances only _____ has London dispersion. CH2CH3 O CHOCH-CH2OH d.
10 M NH 4 NO 3 b. See the answer See the answer done loading. Select the pair of substances in which the one with the higher vapor pressure at a given temperature is listed first.
Correct option is A Boiling point elevation depends on the solvent of course but were assuming water and upon the concentration of the solute and how many particles into which the solute breaks. Which of the following will have the highest boiling point. Of the following _____ has the highest boiling point.
Δ T b i K b m Where Δ T b Boiling point elevation i Vant Hoff factor K b Constant m molality. The higher the Vant Hoff factor i the higher the boiling point. A colligative property depends on the number of particles present in solute present in the given solution.
The ionic molecule with higher charge will have higher boiling point. Which one of the following should have the highest boiling point. The elevation in boiling point is a colligative property.
So the correct option is ii. The boiling point of a molecule depends on its structure. 125 m C6H12O6 b.
Which of the following aqueous solutions has the highest boiling point. Which of the following should have the highest boiling point. The Na2CO3 solution has a lower osmotic pressure and lower vapor pressure than.
The solvent used here is water. Hence 10 M N a2SO4 has highest value of boiling point. Which of the following should have the highest melting point.
Solution should have the highest boiling point. RbCl CH₃Cl CH₃OH CH⁴ stronger dispersion forces Krypton has a higher melting point than argon because of its. Appeared first on nursing assignment tutor.
Relation between the boiling point elevation and the vant hoff factor is as shown below. Arrange the following in order of increasing boiling point. Which one of the following should have the highest boiling point.
The intermolecular forces that are most significant accounting for the high boiling point of liquid water relative to other substances of similar molecular weight are_ ion-ion attraction metallic bonding ion-induced dipole London dispersion forces hydrogen bonding Which of the following liquids should have the highest viscosity at the same temperature. 10 M KNO 3 4. Which of the following should have the highest boiling pointA.
125 m KNO3 c. Of the following substances _____ has the highest boiling point. C 7 H 16 C 5 H 12 B.
N2 Br2 H2 Cl2 O2. H2O CO2 CH4 Kr NH3. Colligative properties like boiling point elevation depend on the total number of particles in solution.
Chemistry questions and answers. CH 3 CH. H 2 O H 2 S D.
ACF4 BCCl4 CCBr4 DCI4 ECH4. For example CBr4 have a higher boiling point than other compounds because they have both London dispersion forces and dipole-dipole interactions. NaCl have ions with charge -1.
So that leaves Na2SO4 and NaCl. 125 m CaNO32 d. A C2H6 b C2H5OH c C2H5F d C2H5CI.

Solved H3ch2ch3 Ch3ch3 E Ch4 48 Which One Of The Following Chegg Com

Which Of The Following Aqueous Solutions Has The Highest Boiling Point
Why Do Larger Alkanes Have Higher Boiling Points Quora

Chemistry Notes Spontaneity Entropy And Gibbs Free Energy Chemistry Notes Chemistry Chemistry Basics

Melting And Boiling Points Of Select Salt Compounds Download Table
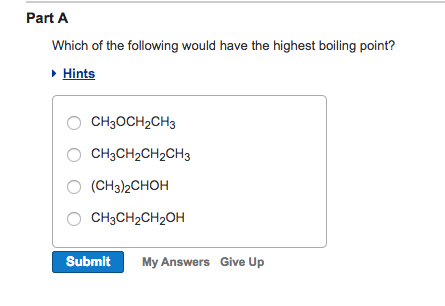
Solved Part A Which Of The Following Would Have The Highest Chegg Com

Chem 3102 Sapling Week 8 Exp 3 3 A B Structural Effects Of Boiling Point And Refractive Index Unknown Liquid Flashcards Quizlet

Chemistry 11 Periodic Table Trend Periodic Table Physical Properties Periodic Table Group 1

Comments
Post a Comment